Benefits of IV Therapy You Never Knew About
Not too long ago, IV therapy could only be applied in a hospital setting and when it was first introduced, it helped people a lot with recovery and other health aspects. Nowadays, IV therapy can be used outside the hospital and it has become available to everyone. The benefits this therapy brings to people are countless and with each year, as technology is being improved, it gets better.

How do they work?
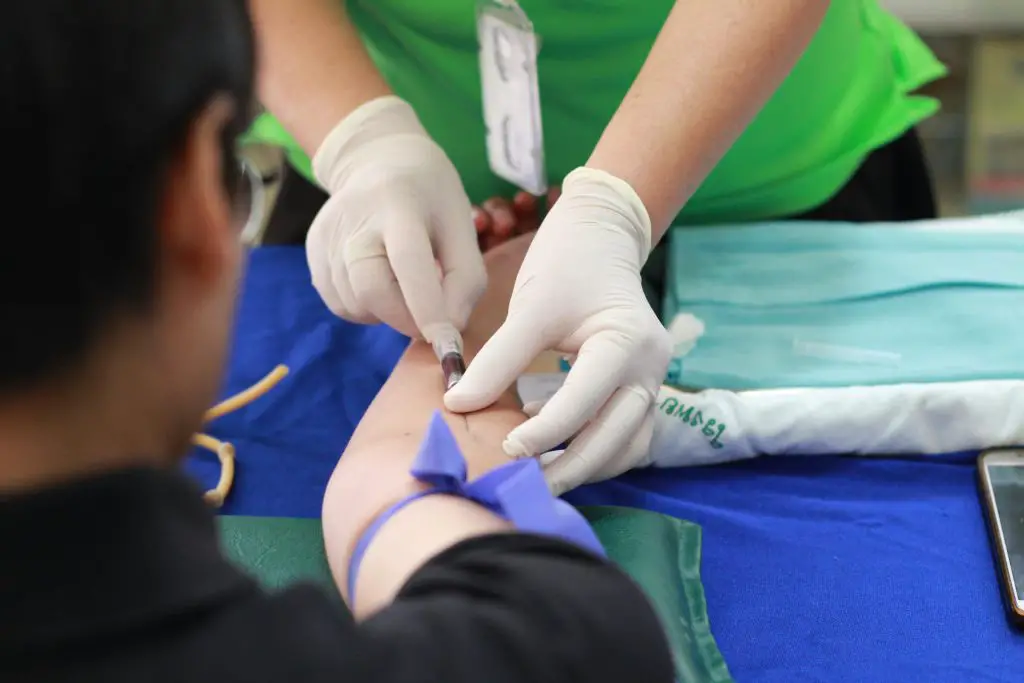
Intravenous therapy or IV therapy delivers nutrients to your body through the veins, as the name suggests. This method is much better than some other ways of getting nutrients into your body, especially when rapid absorption is needed due to your health. The first step is preparing you for the therapy. This involves assessing the patient’s history, health situation, and other factors and then applying the right IV therapy. The medical specialist then chooses a vein in your arm or hand where they will inject you. Of course, they make sure that everything is sterile before they start the procedure. During the procedure, they will monitor the patient’s vital signs and once the therapy is finished, they will carefully remove the needle from the vein and patch everything up. The patient will be monitored for a time the experts think is necessary and then they can continue any other treatments if there is a need for them.
It is available outside the hospital
What many people think is that they have to go to the hospital to get their IV but that is not the case. Most places offer this treatment whenever you need it. If you want to have IV therapy in Albuquerque, for example, you can get many options, including an athletic performance boost, metabolic support, or immune booster. There are many reasons why someone would want to do this treatment this way. Usually, it is much more comfortable to get an IV in a setting where there are not many people around and where you can take your time. In the hospital, you won’t get the same privacy as you would in your home or somewhere in a clinic. Also, if you are very busy and you cannot wait in line in the hospital, by going somewhere where they offer this treatment, you will be done faster and easier. Since the people who give IV treatments usually work for themselves, which means that they earn as much as they work, they are usually nice since you are their customer.
Nutrient absorption
The main goal of IVs is to get nutrients into your system faster than some other available methods. Instead of using oral options, which require that your digestive system absorb the nutrients, which can take some time, and other options IVs will get the nutrients directly into your bloodstream, where they are most needed. This will enable your body to get the nutrients in the fastest way possible, which can often be a lifesaver. Also, when using this treatment, the experts will be able to get your body the right dosage it needs, which is crucial when navigating some illnesses and health conditions.

Rehydrating your body
Water is very important to our body so it is normal that we want to be hydrated at all times. If your body is not properly hydrated, it can lead to many problems. The water in your body regulates the temperature by cooling it, especially during hot days. Also, it transports oxygen and other nutrients to our cells, and without it, they would cease to exist. It also lubricates joints, helps with digestion, and does many other things. If you suffer from dehydration and need to rehydrate as soon as possible, that is when IVs are used. Also, if you are not able to drink anything, the IV will come as a substitute. They contain different electrolytes, potassium, and chloride, which all help our body function properly.
Customized treatment
When experts want to give you an exact amount of nutrients in your body, they will use an IV. Some illnesses are very delicate and they require precise medication. If the patient’s intake is not properly managed, it can lead to a lot of problems. The experts will consider different factors when deciding what dosage they are going to use, depending on age, health, and other major factors.
It raises your energy
Almost everyone who has been sick and has taken an IV knows how much they instantly feel better. IVs are known for having a lot of minerals and vitamins that can boost energy. The treatment injects them directly into your bloodstream, which tackles the fatigue that you may be feeling. So if you ever feel like you can barely get out of bed and you cannot help yourself anymore, you should go to the ER and if they feel that you need an IV, you will get a lot better real soon.
Quicker recovery
IVs are known for helping with recovery, whether it is due to intense physical activity or an illness. When it introduces nutrients into your bloodstream, it helps repair the tissues that have been damaged during physical activities.
Treating illnesses
Experts use IVs to treat some conditions and illnesses and help people recover faster from them. They are frequently used to treat various infections, such as those that require high doses of medication. Also, those that have electrolyte disbalances are treated using them since they have electrolytes, potassium, and chloride. Did you know that chemotherapy is administered intravenously? So IVs can also be used to cure various types of cancer. Various autoimmune disorders and chronic conditions can all be treated using it.
IVs have revolutionized modern medicine, and without them, many illnesses and diseases would be harder to treat and many people would not be saved if they did not exist. The use of IVs is widespread in almost every field of medicine and many people use them outside the hospital to experience relief and boost their energy. As you can see, the benefits of IVs cannot be numbered, and without them, we would be much worse off.

Throughout his career, Andras Kovacs has developed a deep understanding of DNA and its applications in genealogy and genetic testing. He has helped thousands of individuals uncover their ancestral heritage, using cutting-edge DNA analysis to trace family lineages and reveal connections across generations.